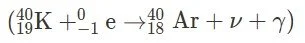GCSE Physics Tutorial - Balancing Radioactive Decay Equations
Introduction: In this tutorial, we will learn how to balance radioactive decay equations. Radioactive decay equations represent the process by which unstable atomic nuclei transform into more stable configurations by emitting various types of radiation. Balancing these equations is crucial in nuclear physics to ensure that the total mass number (A) and atomic number (Z) are conserved on both sides of the equation. Balancing radioactive decay equations correctly allows us to accurately represent the decay process and understand the transformations of unstable nuclei. Let's delve into the key steps for balancing radioactive decay equations.
Steps to Balance Radioactive Decay Equations:
Identify the Decay Mode: The first step is to identify the decay mode from the decay equation. Common decay modes include alpha decay ($ \alpha $), beta-minus decay ($ \beta^- $), beta-plus decay ($ \beta^+ $), gamma decay ($ \gamma $), electron capture ($ \text{EC} $), and positron emission ($ \text{β}^+ $).
Determine the Daughter Nucleus: Next, determine the daughter nucleus that results from the decay process. The daughter nucleus is the resulting nucleus after the decay of the parent nucleus. It may have a different atomic number (Z) and mass number (A) compared to the parent nucleus.
Write the Decay Equation: Write the initial decay equation by placing the parent nucleus on the left-hand side and the daughter nucleus and emitted particle or radiation on the right-hand side. Include the symbols for the respective decay mode and the emitted particles.
Mass Number (A) Conservation: Ensure that the total mass number (A) is conserved on both sides of the equation. The sum of A on the left-hand side (parent nucleus) should be equal to the sum of A on the right-hand side (daughter nucleus and emitted particles).
Atomic Number (Z) Conservation: Ensure that the total atomic number (Z) is conserved on both sides of the equation. The sum of Z on the left-hand side (parent nucleus) should be equal to the sum of Z on the right-hand side (daughter nucleus and emitted particles).
Balance by Adjusting the Emitted Particles: If the equation is not balanced, adjust the number of emitted particles on the right-hand side to balance the equation. For example, if the equation involves the emission of two beta particles, make sure to include two beta particles on the right-hand side.
Verify the Balanced Equation: After making adjustments, verify that the equation is balanced by checking that the total mass number (A) and atomic number (Z) are equal on both sides.
Example Equations:
a. Alpha Decay:
b. Beta-Minus Decay:
c. Beta-Plus Decay:
d. Gamma Decay:
e. Electron Capture:
Conclusion: In this tutorial, we have learned how to balance radioactive decay equations correctly. By identifying the decay mode, determining the daughter nucleus, and ensuring the conservation of mass number (A) and atomic number (Z), we can accurately represent the decay process. Balancing these equations is essential in nuclear physics to understand the transformations of unstable atomic nuclei and their applications in radiometric dating, medical imaging, and nuclear energy.
Looking for a more dynamic learning experience?
Explore our engaging video lessons and interactive animations that GoPhysics has to offer – your gateway to an immersive physics education!