Difference Between the Plum Pudding Model and the Nuclear Model of the Atom
In this tutorial, we’ll explore the key differences between two important atomic models: the Plum Pudding model and the Nuclear model. Both played a major role in the development of atomic theory, but they differ in how they explain the atom’s structure and distribution of charge.
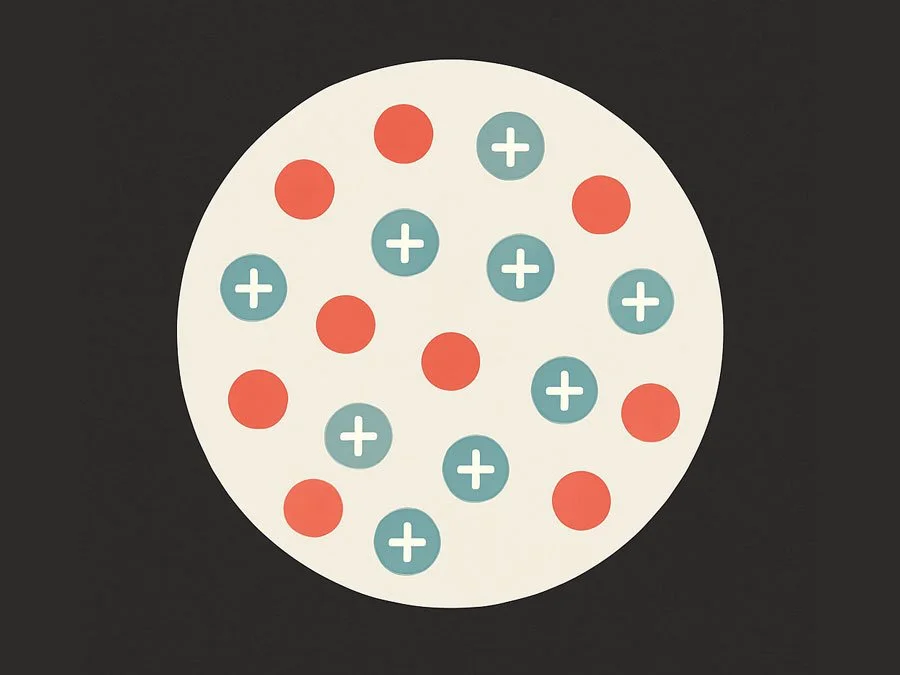
The Plum Pudding Model (J.J. Thomson, late 1800s)
This was one of the earliest models of the atom. According to Thomson:
Structure: The atom was thought to be a soft, positively charged sphere.
Electrons: Electrons were scattered throughout this sphere—like raisins in a plum pudding.
Charge: The positive and negative charges were evenly spread, so the atom was overall neutral.
The Nuclear Model (Ernest Rutherford, early 1900s)
Rutherford’s model was developed after his gold foil experiment, which changed how scientists viewed the atom:
Nucleus: Most of the atom’s mass and positive charge is packed into a small, dense core called the nucleus.
Electrons: Electrons orbit around the nucleus, similar to planets orbiting the sun.
Charge: The positive charge of the nucleus is balanced by the negative electrons—so the atom remains neutral overall.
Key Differences
1. Charge Distribution
Plum Pudding: Charge is spread evenly throughout the atom.
Nuclear Model: Positive charge is concentrated in the centre (nucleus), with electrons surrounding it.
2. Presence of a Nucleus
Plum Pudding: No nucleus—just a uniform sphere of charge.
Nuclear Model: A central nucleus holds most of the atom’s mass and positive charge.
3. Experimental Support
Plum Pudding: Based on theory, with no strong experimental evidence.
Nuclear Model: Supported by Rutherford’s gold foil experiment, which showed that some alpha particles bounced back—impossible if charge was spread evenly.
Summary
The Plum Pudding model suggested that atoms were soft spheres with evenly spread charge. The Nuclear model introduced the idea of a dense, central nucleus, with electrons orbiting around it — an idea backed by experimental evidence. This shift marked a turning point in atomic theory and laid the foundation for the more advanced models we use today.
Related Posts:
The Atomic Model Before the Discovery of the Electron
The Discovery of the Electron and the Plum Pudding Model of the Atom