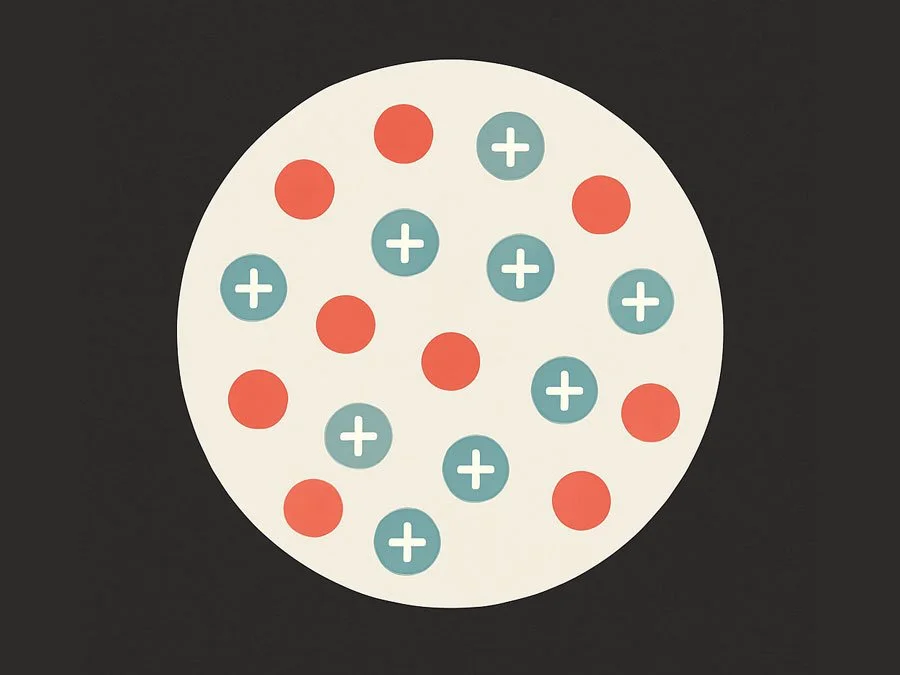
Difference Between the Plum Pudding Model and the Nuclear Model of the Atom
In this tutorial, we’ll explore the key differences between two important atomic models: the Plum Pudding model and the Nuclear model. Both played a major role in the development of atomic theory, but they differ in how they explain the atom’s structure and distribution of charge.
The Plum Pudding Model (J.J. Thomson, late 1800s)
This was one of the earliest models of the atom. According to Thomson:
Structure: The atom was thought to be a soft, positively charged sphere.
Electrons: Electrons were scattered throughout this sphere—like raisins in a plum pudding.
Charge: The positive and negative charges were evenly spread, so the atom was overall neutral.
The Nuclear Model (Ernest Rutherford, early 1900s)
Rutherford’s model was developed after his gold foil experiment, which changed how scientists viewed the atom:
Nucleus: Most of the atom’s mass and positive charge is packed into a small, dense core called the nucleus.
Electrons: Electrons orbit around the nucleus, similar to planets orbiting the sun.
Charge: The positive charge of the nucleus is balanced by the negative electrons—so the atom remains neutral overall.
Key Differences
1. Charge Distribution
Plum Pudding: Charge is spread evenly throughout the atom.
Nuclear Model: Positive charge is concentrated in the centre (nucleus), with electrons surrounding it.
2. Presence of a Nucleus
Plum Pudding: No nucleus—just a uniform sphere of charge.
Nuclear Model: A central nucleus holds most of the atom’s mass and positive charge.
3. Experimental Support
Plum Pudding: Based on theory, with no strong experimental evidence.
Nuclear Model: Supported by Rutherford’s gold foil experiment, which showed that some alpha particles bounced back—impossible if charge was spread evenly.
Summary
The Plum Pudding model suggested that atoms were soft spheres with evenly spread charge. The Nuclear model introduced the idea of a dense, central nucleus, with electrons orbiting around it — an idea backed by experimental evidence. This shift marked a turning point in atomic theory and laid the foundation for the more advanced models we use today.
Related Posts:
The Atomic Model Before the Discovery of the Electron
The Discovery of the Electron and the Plum Pudding Model of the Atom
GCSE Physics Tutorial - Scattering Experiments and the Changing Atomic Model
Introduction: In this tutorial, we will explore how new evidence from scattering experiments led to significant changes in the atomic model. Scientists conducted these experiments to investigate the structure of the atom and the behaviour of subatomic particles. The results of these experiments challenged existing atomic models and paved the way for a deeper understanding of atomic structure. Let's delve into the key experiments and the impact they had on shaping the atomic model.
Background: Before the advent of modern atomic models, the prevailing model was the Plum Pudding model, proposed by J.J. Thomson. According to this model, the atom was considered a positively charged sphere with electrons dispersed throughout.
The Gold Foil Experiment: Ernest Rutherford and his colleagues conducted the gold foil experiment, also known as the alpha particle scattering experiment. In this experiment, they directed alpha particles (positively charged particles) at a thin sheet of gold foil.
Unexpected Results: Contrary to expectations based on the Plum Pudding model, Rutherford observed that some alpha particles were deflected at large angles, and a few even bounced straight back. This indicated that most of the atom's mass and positive charge were concentrated in a tiny, dense region at the centre, which Rutherford named the nucleus.
Rutherford's Nuclear Model: Based on the experimental results, Rutherford proposed a new atomic model called the "nuclear model" or "planetary model." According to this model: a. The nucleus: The majority of the atom's mass and positive charge are concentrated in the nucleus, which is tiny compared to the overall size of the atom. b. Electrons: Electrons, being much lighter and negatively charged, revolve around the nucleus at significant distances.
The Discovery of Protons: Subsequent experiments by other scientists led to the discovery of protons, positively charged particles within the nucleus. This discovery further supported the nuclear model and provided evidence that the positive charge of the nucleus could be subdivided into smaller particles.
The Discovery of Neutrons: Experiments by James Chadwick revealed the existence of neutrons, neutral particles also present in the nucleus. This discovery completed the understanding of atomic nuclei as composed of protons and neutrons, with electrons orbiting around the nucleus.
Quantum Mechanics and Modern Atomic Models: The development of quantum mechanics in the 20th century provided a deeper understanding of the behaviour of subatomic particles and their interaction within atoms. Quantum mechanics laid the foundation for the modern atomic model, which incorporates wave-particle duality and the concept of atomic orbitals.
Conclusion: In this tutorial, we have explored how new evidence from scattering experiments led to significant changes in the atomic model. Rutherford's gold foil experiment revealed the presence of a positively charged nucleus at the centre of the atom, leading to the nuclear model. The discovery of protons and neutrons within the nucleus further refined the model and completed the understanding of atomic nuclei. Subsequent developments in quantum mechanics paved the way for the modern atomic model, which considers the dual nature of particles and the behaviour of electrons within atomic orbitals. These scattering experiments and the changing atomic model have revolutionised our understanding of atomic structure, laying the foundation for further advancements in nuclear physics and quantum mechanics.
Looking for a more dynamic learning experience?
Explore our engaging video lessons and interactive animations that GoPhysics has to offer – your gateway to an immersive physics education!
GCSE Physics Tutorial - Discovery of Neutrons by James Chadwick
In this tutorial, we will explore the experimental work of James Chadwick, which provided crucial evidence for the existence of neutrons within the atomic nucleus. Chadwick's groundbreaking discovery came about 20 years after the concept of the atomic nucleus was accepted in scientific circles. Let's delve into the key experiments and insights that led to the identification of neutrons as another essential constituent of the atomic nucleus.
Background: Following Ernest Rutherford's discovery of the atomic nucleus in the early 20th century, scientists sought to understand the nature of the positively charged protons within the nucleus. However, there was a discrepancy between the mass of the nucleus, as determined by the total number of protons, and its observed mass from experiments.
The Mass Defect: Experiments showed that the mass of a nucleus was slightly less than the sum of the masses of its individual protons and electrons. This discrepancy became known as the "mass defect."
Chadwick's Experiments: In the early 1930s, James Chadwick conducted experiments to investigate the origin of the mass defect and provide a more complete understanding of the atomic nucleus.
The Discovery of Neutrons: Chadwick's key experiments involved bombarding beryllium with alpha particles. He observed that the alpha particles were scattered, and additional radiation was produced. This additional radiation was neutral and had a mass slightly larger than a proton, consistent with the mass defect.
Neutron Emission: Chadwick concluded that the additional radiation emitted during the beryllium-alpha particle collisions consisted of neutral particles with a mass similar to that of a proton. He named these neutral particles "neutrons."
Neutrons and the Mass Defect: Chadwick's discovery of neutrons explained the mass defect observed in nuclear experiments. The neutrons accounted for the missing mass and played a crucial role in balancing the positive charges of protons in the nucleus.
Electrical Neutrality of Neutrons: Neutrons carry no electrical charge, making them electrically neutral. Unlike protons and electrons, which carry positive and negative charges, respectively, neutrons have no net charge.
Significance of Chadwick's Discovery: Chadwick's discovery of neutrons solidified the understanding of atomic nuclei as composed of protons and neutrons, with electrons orbiting around the nucleus. This discovery further enhanced the nuclear model of the atom, providing a more comprehensive picture of atomic structure.
Later Contributions: Chadwick's discovery of neutrons opened the door for further research into nuclear physics and led to the development of nuclear energy and modern particle physics.
In this tutorial, we have explored the experimental work of James Chadwick, which provided crucial evidence for the existence of neutrons within the atomic nucleus. Chadwick's discovery, about 20 years after the acceptance of the atomic nucleus, filled the gap in understanding the mass defect and unveiled the presence of neutral particles, the neutrons, in the nucleus. This breakthrough advanced our understanding of atomic structure and laid the groundwork for further research in nuclear physics and particle physics. The discovery of neutrons, along with protons and electrons, as fundamental constituents of the atomic nucleus remains a cornerstone of modern atomic theory.
Looking for a more dynamic learning experience?
Explore our engaging video lessons and interactive animations that GoPhysics has to offer – your gateway to an immersive physics education!
GCSE Physics Tutorial - The Subdivision of Nucleus Positive Charge: Discovery of Protons
In this tutorial, we will explore how experiments conducted after the Bohr model led to the revolutionary idea that the positive charge of any atomic nucleus could be subdivided into smaller particles. This discovery marked a significant advancement in our understanding of atomic structure and paved the way for further insights into the constituents of the atomic nucleus. Let's delve into the key experiments and ideas that led to the identification of these smaller positively charged particles.
Background: After Niels Bohr proposed his atomic model, which described the arrangement of electrons in discrete energy levels, scientists continued to investigate the structure of the atomic nucleus. They sought to understand the nature of the positively charged nucleus and its role in defining the properties of different elements.
The Discovery of Protons: The key experiments that led to the idea of subdividing the positive charge of the nucleus involved the study of radioactivity and the interaction of particles with matter. In the early 20th century, Ernest Rutherford conducted experiments involving alpha particles, which are positively charged particles emitted during certain types of radioactive decay.
Rutherford's Gold Foil Experiment: In Rutherford's famous gold foil experiment, alpha particles were directed at a thin sheet of gold foil. According to the prevailing Plum Pudding model, the alpha particles were expected to pass through the gold foil with only minor deflections due to the uniform distribution of positive charge in the atom.
Unexpected Results: Contrary to expectations, some of the alpha particles experienced significant deflections, while a few even bounced directly backward. This unexpected outcome indicated that most of the atom's mass and positive charge were concentrated in a tiny, dense region at the center, which Rutherford named the nucleus.
Conclusions: Based on the results of the gold foil experiment and subsequent research, it became clear that the positive charge of the nucleus was not uniformly distributed but was concentrated in individual positively charged particles. These particles were named "protons."
Protons: Elementary Unit of Positive Charge: Protons are elementary particles carrying a positive charge. Each proton has an electric charge of +1 elementary charge, denoted as "e." The charge of one proton is equal in magnitude but opposite in sign to the charge of one electron, which has a charge of -1e.
Atomic Number and Protons: The number of protons present in an atom's nucleus is known as the atomic number (Z). The atomic number defines the identity of the element, as atoms of different elements have different numbers of protons. For example, all carbon atoms have six protons in their nucleus, resulting in a carbon atom having an atomic number of 6.
Electrical Neutrality of Atoms: Atoms are electrically neutral, meaning they have an equal number of protons (positive charge) and electrons (negative charge). The positive charge of the protons is balanced by the negative charge of the electrons, resulting in no net charge for the atom as a whole.
Conclusion: In this tutorial, we have explored how experiments conducted after the Bohr model led to the groundbreaking idea that the positive charge of any atomic nucleus could be subdivided into smaller particles, known as protons. The discovery of protons provided crucial evidence that the nucleus contained individual positively charged entities, each carrying the elementary unit of positive charge. This revelation significantly advanced our understanding of atomic structure and laid the foundation for further research in nuclear physics and quantum mechanics. The identification of protons as one of the fundamental building blocks of matter continues to be a cornerstone in modern atomic theory.
Recalling the Discovery of the Neutron
In the early 20th century, the understanding of atomic structure was evolving rapidly due to groundbreaking experiments and discoveries. One of the significant discoveries was the existence of the neutron, a subatomic particle that plays a crucial role in the composition of atomic nuclei.
The Search for the Neutron: At the time, it was known that atoms were composed of protons, electrons, and a nucleus. However, there were some inconsistencies in the atomic model. For instance, the mass of an atom's nucleus was significantly larger than the combined masses of its protons and electrons. This led scientists to hypothesize the existence of another subatomic particle within the nucleus.
James Chadwick's Experiment: In 1932, British physicist James Chadwick conducted an experiment that provided strong evidence for the existence of the neutron. Chadwick used a technique known as "scattering" to investigate the behaviour of particles when they collided with atoms. He bombarded beryllium atoms with alpha particles, which are positively charged particles commonly emitted during radioactive decay.
Chadwick observed that the scattering of alpha particles by beryllium atoms produced an uncharged particle that had roughly the same mass as a proton. This particle was initially called the "neutral proton" but was later named the "neutron." The discovery of the neutron provided a more complete understanding of atomic nuclei and resolved the inconsistency in the mass of atomic nuclei.
Key Points to Remember:
Neutron's Charge: Unlike protons and electrons, neutrons have no electric charge. They are electrically neutral particles.
Mass of Neutron: The mass of a neutron is slightly larger than that of a proton.
Stability of Nuclei: The presence of neutrons in atomic nuclei helps stabilise them by counteracting the repulsive forces between positively charged protons. Neutrons contribute to the strong nuclear force that holds the nucleus together.
Isotopes: The number of neutrons in an atom's nucleus can vary while keeping the number of protons constant. Atoms of the same element with different numbers of neutrons are called isotopes.
Significance: The discovery of the neutron had a profound impact on the understanding of atomic structure and the behaviour of matter. It paved the way for further research into nuclear physics and led to the development of technologies such as nuclear reactors and nuclear weapons. The neutron's presence and its interactions with other particles play a critical role in nuclear reactions and processes.
In summary, the discovery of the neutron was a milestone in the field of particle physics, contributing to the refined understanding of atomic nuclei and leading to advancements in various scientific and technological applications.
Looking for a more dynamic learning experience?
Explore our engaging video lessons and interactive animations that GoPhysics has to offer – your gateway to an immersive physics education!
GCSE Physics Tutorial - Discovery of the Neutron
In the early 20th century, the understanding of atomic structure was evolving rapidly due to groundbreaking experiments and discoveries. One of the significant discoveries was the existence of the neutron, a subatomic particle that plays a crucial role in the composition of atomic nuclei.
The Search for the Neutron: At the time, it was known that atoms were composed of protons, electrons, and a nucleus. However, there were some inconsistencies in the atomic model. For instance, the mass of an atom's nucleus was significantly larger than the combined masses of its protons and electrons. This led scientists to hypothesize the existence of another subatomic particle within the nucleus.
James Chadwick's Experiment: In 1932, British physicist James Chadwick conducted an experiment that provided strong evidence for the existence of the neutron. Chadwick used a technique known as "scattering" to investigate the behaviour of particles when they collided with atoms. He bombarded beryllium atoms with alpha particles, which are positively charged particles commonly emitted during radioactive decay.
Chadwick observed that the scattering of alpha particles by beryllium atoms produced an uncharged particle that had roughly the same mass as a proton. This particle was initially called the "neutral proton" but was later named the "neutron." The discovery of the neutron provided a more complete understanding of atomic nuclei and resolved the inconsistency in the mass of atomic nuclei.
Key Points to Remember:
Neutron's Charge: Unlike protons and electrons, neutrons have no electric charge. They are electrically neutral particles.
Mass of Neutron: The mass of a neutron is slightly larger than that of a proton.
Stability of Nuclei: The presence of neutrons in atomic nuclei helps stabilise them by counteracting the repulsive forces between positively charged protons. Neutrons contribute to the strong nuclear force that holds the nucleus together.
Isotopes: The number of neutrons in an atom's nucleus can vary while keeping the number of protons constant. Atoms of the same element with different numbers of neutrons are called isotopes.
Significance: The discovery of the neutron had a profound impact on the understanding of atomic structure and the behaviour of matter. It paved the way for further research into nuclear physics and led to the development of technologies such as nuclear reactors and nuclear weapons. The neutron's presence and its interactions with other particles play a critical role in nuclear reactions and processes.
In summary, the discovery of the neutron was a milestone in the field of particle physics, contributing to the refined understanding of atomic nuclei and leading to advancements in various scientific and technological applications.
Looking for a more dynamic learning experience?
Explore our engaging video lessons and interactive animations that GoPhysics has to offer – your gateway to an immersive physics education!
GCSE Physics Tutorial - Niels Bohr's Model: Electrons Orbiting the Nucleus at Specific Distances
In this tutorial, we will explore Niels Bohr's atomic model, which revolutionised our understanding of atomic structure. Bohr proposed that electrons orbit the nucleus at specific distances in well-defined energy levels. His model addressed the limitations of previous models and provided key insights into the behaviour of electrons within atoms. Let's delve into the main features of Bohr's model and its significance in modern atomic theory.
The Bohr Model of the Atom: Niels Bohr, a Danish physicist, proposed his atomic model in 1913, building on Ernest Rutherford's nuclear model. Bohr's model introduced the concept of quantised energy levels for electrons, resolving some of the issues with Rutherford's model.
Energy Levels and Orbits: Bohr suggested that electrons exist in specific energy levels or orbits around the nucleus. Each energy level has a fixed energy value, and electrons can only occupy these allowed orbits.
Quantisation of Energy: Bohr's key insight was that electrons can gain or lose energy by jumping between energy levels. When an electron absorbs energy, it jumps to a higher energy level (excited state), and when it releases energy, it falls back to a lower energy level (ground state).
Stability and Radiation: Bohr's model explained why electrons in stable atoms do not continuously emit radiation as they orbit the nucleus. Electrons in stable orbits are in their lowest energy states and do not radiate energy.
Absorption and Emission Spectra: Bohr's model successfully explained the patterns observed in atomic absorption and emission spectra. When an electron jumps between energy levels, it emits or absorbs energy in the form of discrete packets called photons.
Bohr's Postulates: Bohr's model was based on three key postulates: a. Electrons move in circular orbits around the nucleus at specific distances, known as energy levels. b. Electrons do not radiate energy while in stable orbits. c. Electrons can absorb or emit energy when transitioning between energy levels.
Limitations and Quantum Mechanics: While Bohr's model was groundbreaking, it had limitations. It could not explain the behaviour of atoms with more than one electron. The development of quantum mechanics in the 1920s further refined our understanding of atomic structure and electron behaviour.
Legacy and Impact: Bohr's model marked a significant advancement in atomic theory and provided a bridge between classical physics and quantum mechanics. It laid the foundation for the study of atomic physics and inspired further research into the quantum nature of matter.
In this tutorial, we have explored Niels Bohr's atomic model, which proposed that electrons orbit the nucleus at specific distances in quantised energy levels. Bohr's model addressed the limitations of previous models and provided key insights into the behaviour of electrons within atoms. His work marked a significant step in the development of modern atomic theory and inspired further research in quantum mechanics. While Bohr's model has been refined with the advent of quantum physics, it remains a pivotal contribution to our understanding of the fascinating world of atoms and their behavior.
Looking for a more dynamic learning experience?
Explore our engaging video lessons and interactive animations that GoPhysics has to offer – your gateway to an immersive physics education!
GCSE Physics Tutorial - The Alpha Particle Scattering Experiment and the Discovery of the Nucleus
In this tutorial, we will explore the alpha particle scattering experiment and how it led to the groundbreaking conclusion that the mass of an atom is concentrated at the center, known as the nucleus, and that the nucleus is positively charged. The experiment, conducted by Ernest Rutherford and his colleagues, provided crucial evidence that revolutionised our understanding of atomic structure. Let's delve into the key features of the experiment and its significance in the development of modern atomic theory.
Background: Before the alpha particle scattering experiment, the prevailing atomic model was the Plum Pudding model proposed by J.J. Thomson. According to this model, the atom was envisioned as a positively charged sphere with electrons dispersed throughout.
The Experiment: In 1909, Ernest Rutherford, along with his colleagues Hans Geiger and Ernest Marsden, conducted the alpha particle scattering experiment. They directed alpha particles (positively charged particles) at a thin sheet of gold foil.
The Expected Outcome: Based on the Plum Pudding model, they expected the alpha particles to pass through the gold foil with only slight deflections due to the evenly distributed positive charge.
The Surprising Results: Contrary to expectations, Rutherford and his team observed some alpha particles being deflected at large angles, and a few even bounced straight back. This result was unexpected and challenged the existing atomic model.
Rutherford's Conclusions: Based on the experimental results, Rutherford proposed a new atomic model, known as the "nuclear model" or "planetary model." a. The nucleus: Rutherford concluded that most of the atom's mass and positive charge are concentrated at the center, called the nucleus. The nucleus is tiny compared to the overall size of the atom. b. The electrons: Electrons, being much lighter and negatively charged, revolve around the nucleus at significant distances.
Significance of the Nuclear Model: The alpha particle scattering experiment provided evidence for the existence of a tiny, dense, positively charged nucleus at the centre of the atom. This model addressed the limitations of the Plum Pudding model and introduced a new understanding of atomic structure.
Subsequent Discoveries: Rutherford's nuclear model set the stage for further research, leading to the discovery of the neutron (a neutral particle) by James Chadwick in 1932. This completed the modern picture of the atom, with the nucleus consisting of protons (positively charged) and neutrons.
Legacy and Impact: The alpha particle scattering experiment and the nuclear model laid the foundation for modern atomic theory. The concept of the nucleus and the understanding of subatomic particles revolutionised our understanding of matter and paved the way for advancements in nuclear physics.
In this tutorial, we have explored the alpha particle scattering experiment conducted by Ernest Rutherford, which led to the groundbreaking conclusion that the mass of an atom is concentrated at the centre (nucleus) and that the nucleus is positively charged. Rutherford's discovery challenged the prevailing Plum Pudding model and introduced the nuclear model of the atom. The experiment's significance lies in providing crucial evidence for the existence of the nucleus and its positive charge, which revolutionised our understanding of atomic structure. The alpha particle scattering experiment remains a pivotal moment in the history of atomic physics, guiding further research and advancements in our quest to comprehend the fundamental building blocks of matter.
Looking for a more dynamic learning experience?
Explore our engaging video lessons and interactive animations that GoPhysics has to offer – your gateway to an immersive physics education!
GCSE Physics Tutorial - The Discovery of the Electron and the Plum Pudding Model of the Atom
In this tutorial, we will explore the discovery of the electron and how it led to the development of the Plum Pudding model of the atom. The discovery of the electron revolutionised our understanding of atomic structure and played a crucial role in shaping early atomic models. Let's delve into the key experiments and ideas that paved the way for the Plum Pudding model.
The Discovery of the Electron: The discovery of the electron is attributed to J.J. Thomson, a British physicist, who conducted experiments with cathode rays in the late 19th century. Cathode rays are streams of electrons emitted from a cathode when an electric current is passed through a vacuum tube.
Thomson's Experiment: In 1897, Thomson conducted experiments using cathode ray tubes and observed the following: a. Cathode rays were deflected by both electric and magnetic fields, indicating that they were charged particles. b. The degree of deflection of cathode rays by the electric field suggested that the particles were much lighter than atoms. c. The deflection of cathode rays by a magnetic field showed that the particles were negatively charged.
Thomson's Conclusions: Based on his experiments, Thomson concluded that cathode rays were composed of negatively charged particles, which he called "corpuscles." These particles are now known as electrons. Thomson's discovery of the electron challenged the prevailing understanding of atomic structure at the time.
The Plum Pudding Model: Building on the discovery of the electron, J.J. Thomson proposed the Plum Pudding model of the atom in 1904. According to this model: a. The atom was considered a sphere of positive charge, like a "plum pudding." b. Electrons (the newly discovered negatively charged particles) were embedded within the positively charged sphere, like "raisins" in a plum pudding.
Significance of the Plum Pudding Model: The Plum Pudding model was the first atomic model to incorporate the existence of electrons. It provided a new perspective on the atom's structure by recognising the presence of negatively charged particles within an overall positively charged sphere.
Limitations of the Plum Pudding Model: While the Plum Pudding model was an important step forward, it had limitations. It couldn't explain the precise arrangement of electrons or the stability of the atom. Later experiments, notably Ernest Rutherford's gold foil experiment, revealed the presence of a small, dense, positively charged nucleus at the center of the atom, leading to the development of the nuclear model.
Legacy and Advancements: The discovery of the electron and the Plum Pudding model marked significant milestones in the history of atomic theory. They set the stage for further research and experiments that ultimately led to the modern understanding of atomic structure and the development of quantum mechanics.
In this tutorial, we have explored how the discovery of the electron by J.J. Thomson led to the development of the Plum Pudding model of the atom. Thomson's experiments with cathode rays provided evidence of the existence of electrons, leading to a new perspective on atomic structure. The Plum Pudding model was an early atomic model that incorporated electrons as negatively charged particles embedded within a positively charged sphere. While this model had limitations and was eventually replaced, it played a crucial role in advancing our understanding of the atom's constituents and their behaviour. The discovery of the electron and the development of the Plum Pudding model laid the foundation for further research and advancements in atomic physics, contributing to our present-day understanding of the fascinating world of atoms and their behaviour.
Looking for a more dynamic learning experience?
Explore our engaging video lessons and interactive animations that GoPhysics has to offer – your gateway to an immersive physics education!
GCSE Physics Tutorial - The Atomic Model before the Discovery of the Electron
In this tutorial, we will recall the atomic model that existed before the discovery of the electron. This model, known as the "Plum Pudding" model, provided an early understanding of the atom's structure. The Plum Pudding model was proposed in the late 19th century by J.J. Thomson and laid the groundwork for future discoveries that revolutionised our understanding of atomic structure. Let's explore the key features of this model and how it shaped our understanding of atoms at the time.
The Plum Pudding Model: The Plum Pudding model, also known as the "raisin cake" model, was proposed in 1904 by J.J. Thomson, a British physicist. According to this model, the atom was envisioned as a positively charged sphere with electrons (negatively charged particles) embedded within it, resembling raisins in a plum pudding.
Thomson's Experiment: Thomson's model was based on his experiments with cathode rays, which were streams of electrons emitted from a cathode when an electric current was passed through a vacuum tube. He observed that cathode rays were deflected by both electric and magnetic fields, suggesting that they were negatively charged particles.
Key Features of the Plum Pudding Model: a. Positive Sphere: The atom was considered a sphere with a positive charge distributed uniformly throughout its volume. b. Embedded Electrons: Electrons, which were newly discovered particles at the time, were believed to be scattered throughout the positively charged sphere.
Limitations of the Plum Pudding Model: The Plum Pudding model could not explain certain phenomena, such as the precise arrangement of electrons and their stability within the atom. The model also did not account for the presence of a central nucleus, which was later discovered.
Advancements and the Discovery of the Nucleus: The Plum Pudding model was short-lived, as subsequent experiments, notably Ernest Rutherford's gold foil experiment in 1911, provided evidence for the existence of a tiny, dense, positively charged nucleus at the center of the atom. This discovery led to the development of the nuclear model of the atom.
Significance and Legacy: While the Plum Pudding model had limitations and was eventually replaced, it was a critical stepping stone in the journey to unraveling the atom's structure. It highlighted the presence of negatively charged particles (electrons) and paved the way for further research and groundbreaking discoveries in atomic physics.
In this tutorial, we have recalled the Plum Pudding model, the atomic model that existed before the discovery of the electron. Proposed by J.J. Thomson, this model depicted the atom as a positively charged sphere with electrons dispersed throughout. While the Plum Pudding model had its limitations, it played a crucial role in sparking interest in atomic research and set the stage for subsequent discoveries that revolutionised our understanding of atomic structure. As science advanced, the Plum Pudding model gave way to more accurate models, such as the nuclear model, which incorporated a central nucleus. The evolution of atomic models reflects the ever-changing nature of scientific exploration and our continuous quest to comprehend the fundamental building blocks of matter.
Looking for a more dynamic learning experience?
Explore our engaging video lessons and interactive animations that GoPhysics has to offer – your gateway to an immersive physics education!
GCSE Physics Tutorial - The Dynamic Nature of Physics: New Evidence Leading to Changed Models
In this tutorial, we will appreciate the dynamic nature of physics, where new evidence and discoveries can lead to changes or replacements of existing scientific models. Physics is a constantly evolving field that seeks to understand the fundamental laws and principles governing the universe. As new evidence emerges, scientific models are refined, modified, or even replaced to better explain natural phenomena. Let's explore how the scientific method and critical thinking drive advancements in physics!
The Scientific Method: The scientific method is a systematic approach used in physics and other sciences to investigate natural phenomena. It involves the following steps: a. Observation: Scientists observe and gather data about a particular phenomenon. b. Hypothesis: Based on observations, scientists formulate a hypothesis to explain the observed behaviour. c. Experimentation: Scientists conduct experiments and tests to gather further data and verify the hypothesis. d. Analysis: The collected data is analysed to draw conclusions about the validity of the hypothesis. e. Conclusion: The results are used to support or reject the hypothesis.
Evolution of Models: As new data and evidence are accumulated through research and experimentation, scientific models may need to be updated or revised. This is a normal part of scientific progress, allowing us to improve our understanding of the natural world.
Examples of Model Evolution: a. Classical Mechanics to Quantum Mechanics: In the early 20th century, quantum mechanics revolutionised the understanding of particles at the atomic and subatomic levels. It replaced classical mechanics for such small-scale phenomena. b. Geocentric to Heliocentric Model: The heliocentric model proposed by Nicolaus Copernicus replaced the geocentric model, which had placed the Earth at the center of the universe. c. The Expanding Universe: The discovery of the expanding universe led to the development of the Big Bang theory, which replaced the steady-state model of the universe.
Importance of Peer Review: Scientific findings are subject to peer review, where other experts in the field evaluate and scrutinise the research and its methodologies. This process ensures the credibility and reliability of scientific discoveries.
The Role of Technology: Advancements in technology, such as powerful telescopes, particle accelerators, and computational simulations, contribute to gathering more precise and detailed data, leading to new insights and potential model updates.
Embracing Uncertainty: Science acknowledges that our understanding of the universe is not absolute, and new evidence may challenge existing theories. Embracing uncertainty drives scientists to explore and question, leading to further discoveries.
In this tutorial, we have appreciated the dynamic nature of physics, where new evidence and discoveries can lead to changes or replacements of existing scientific models. The scientific method, critical thinking, and peer review play crucial roles in driving advancements in physics. Embracing uncertainty and being open to change are vital aspects of scientific progress. Through continuous exploration and the quest for knowledge, physicists seek to uncover the fundamental principles that govern the universe. Keep exploring the fascinating world of physics, as each discovery brings us closer to understanding the wonders of the cosmos.
Looking for a more dynamic learning experience?
Explore our engaging video lessons and interactive animations that GoPhysics has to offer – your gateway to an immersive physics education!