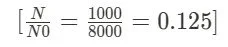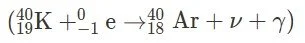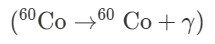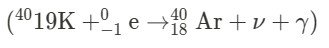GCSE Physics Tutorial - Determining the Half-Life of a Radioactive Isotope
In this tutorial, we will learn how to determine the half-life of a radioactive isotope from given information. The half-life is a fundamental property that describes the decay rate of unstable atomic nuclei. Understanding how to calculate the half-life is crucial in nuclear physics and radiometric dating. Let's delve into the steps involved in determining the half-life of a radioactive isotope.
Steps to Determine the Half-Life of a Radioactive Isotope:
Gather Given Information: Collect all the relevant information provided in the problem. This may include the initial number of radioactive nuclei (N0), the remaining number of radioactive nuclei (N), and the time elapsed (t) between the initial measurement and the current measurement.
Calculate the Fraction of Remaining Nuclei: The fraction of remaining nuclei ($ \frac{N}{N0} )$ is obtained by dividing the number of remaining nuclei (N) by the initial number of nuclei (N0).
Calculate the Decay Constant (λ): The decay constant (λ) is a constant unique to each radioactive isotope and determines the probability of decay per unit time. It can be calculated using the formula: $[ \lambda = \frac{-\ln(\frac{N}{N0})}{t} ]$
Calculate the Half-Life (t½): The half-life (t½) can be determined using the decay constant (λ) with the formula: $[ t_{1/2} = \frac{\ln(2)}{\lambda} ]$
Round the Result: Round the calculated half-life to an appropriate number of significant figures based on the given data and the level of accuracy required.
Example: Let's work through an example to determine the half-life of a hypothetical radioactive isotope:
Suppose an initial sample contains 8000 radioactive nuclei. After 10 days, the number of remaining nuclei is 1000.
Given information:
N0 (Initial number of nuclei) = 8000
N (Remaining number of nuclei) = 1000
t (Time elapsed) = 10 days
Step 1: Calculate the Fraction of Remaining Nuclei:
Step 2: Calculate the Decay Constant (λ):
Step 3: Calculate the Half-Life (t½):
Step 4: Round the Result: The half-life of the radioactive isotope is approximately 3.01 days.
In this tutorial, we have learned how to determine the half-life of a radioactive isotope using given information. By calculating the fraction of remaining nuclei and the decay constant, we can find the half-life of the isotope. This knowledge is valuable in radiometric dating and helps us understand the decay rate of radioactive isotopes, which is essential in various fields of science and technology.
Looking for a more dynamic learning experience?
Explore our engaging video lessons and interactive animations that GoPhysics has to offer – your gateway to an immersive physics education!
GCSE Physics Tutorial - Half-Life and Its Relation to the Random Nature of Radioactive Decay
In this tutorial, we will explore the concept of half-life and how it is related to the random nature of radioactive decay. The half-life of a radioactive isotope is a fundamental property used to describe the decay rate of unstable atomic nuclei. Understanding the relationship between half-life and the random nature of decay is crucial in nuclear physics and radiometric dating. Let's delve into the concept of half-life and how it is influenced by the random behaviour of radioactive decay.
Definition of Half-Life: The half-life of a radioactive isotope is the time it takes for half of the original number of radioactive nuclei in a sample to decay. It is a characteristic property of each radioactive isotope and remains constant over time, regardless of the size of the sample. The concept of half-life is used to describe the exponential decay of radioactive nuclei in a given sample.
Random Nature of Radioactive Decay: Radioactive decay is a random process that occurs at the level of individual atomic nuclei. Unstable atomic nuclei transform into more stable configurations by emitting various types of radiation, such as alpha particles, beta particles, or gamma rays. The timing of decay for an individual nucleus is unpredictable and not influenced by external factors.
Influence of Half-Life on Decay Rate: The half-life of a radioactive isotope is related to the probability of decay for each individual nucleus in the sample. The decay process is probabilistic, meaning that each nucleus has a certain probability of decaying within a specific time interval.
Predicting Individual Decay Times: Due to the random nature of decay, it is not possible to predict when an individual nucleus will decay. However, we can make statistical predictions about the behaviour of a large group of radioactive nuclei. For example, after one half-life, on average, half of the radioactive nuclei in the sample will have decayed.
Consistency of Half-Life: Despite the random nature of decay, the half-life of a radioactive isotope remains constant over time. It does not change based on the number of remaining nuclei or external conditions. This consistency allows scientists to use the concept of half-life to estimate the age of rocks, fossils, and artifacts in radiometric dating.
Half-Life and Radioactive Dating: In radiometric dating, scientists measure the remaining amount of a radioactive isotope in a sample and compare it to the initial amount. By knowing the half-life of the isotope, they can determine the time that has elapsed since the sample was formed.
In this tutorial, we have explored the concept of half-life and its relation to the random nature of radioactive decay. The half-life of a radioactive isotope is the time it takes for half of the radioactive nuclei in a sample to decay, and it remains constant over time. The random nature of decay makes it impossible to predict the exact timing of individual decays, but statistical predictions can be made for a large group of nuclei. Understanding the concept of half-life is essential for radiometric dating and provides valuable information about the decay rate of radioactive isotopes.
Looking for a more dynamic learning experience?
Explore our engaging video lessons and interactive animations that GoPhysics has to offer – your gateway to an immersive physics education!
GCSE Physics Tutorial - The Half-Life of Radioactive Isotopes
In this tutorial, we will explore the concept of the half-life of a radioactive isotope. The half-life is a fundamental characteristic of radioactive decay and is defined as the time it takes for the number of isotope nuclei in a sample to halve. Understanding the half-life is crucial in nuclear physics and has practical applications in radiometric dating and the study of radioactive materials. Let's delve into the definition and significance of the half-life of radioactive isotopes.
Definition of Half-Life: The half-life of a radioactive isotope is the time required for half of the original number of radioactive nuclei in a sample to decay. It is a characteristic property of each radioactive isotope and remains constant over time, regardless of the size of the sample.
Decay and Half-Life: During radioactive decay, unstable atomic nuclei transform into more stable configurations by emitting radiation. The decay process follows an exponential decay curve. Each isotope has a specific half-life, which determines the rate at which its radioactive nuclei decay.
Representing Half-Life: Half-life is usually denoted by the symbol $ t_{1/2} $. It is the time interval in which half of the radioactive nuclei in the sample have decayed. After one half-life, the number of radioactive nuclei remaining in the sample is reduced by half.
Example of Half-Life: Suppose we have a sample of a radioactive isotope with a half-life of 10 minutes. If we start with 1000 nuclei at the beginning, after 10 minutes, 500 nuclei will remain. After another 10 minutes (20 minutes in total), only 250 nuclei will remain, and so on.
Half-Life is Independent of Sample Size: The half-life of a radioactive isotope remains constant regardless of the size of the sample. Whether the sample contains a large number of radioactive nuclei or just a few, the time taken for half of the nuclei to decay remains the same.
Applications of Half-Life: a. Radiometric Dating: By measuring the remaining amount of a radioactive isotope and knowing its half-life, scientists can estimate the age of rocks, fossils, and artifacts. This method is used in archaeology, geology, and paleontology. b. Medical Applications: In nuclear medicine, the half-life of radioactive isotopes is used to determine the appropriate dosage and timing for medical imaging and radiation therapy. c. Nuclear Power: Understanding the half-life of radioactive isotopes is essential for managing and safely disposing of nuclear waste generated in nuclear power plants.
In this tutorial, we have explored the concept of the half-life of radioactive isotopes. The half-life is the time it takes for half of the radioactive nuclei in a sample to decay, and it remains constant for each isotope. Understanding the half-life is crucial for various applications in radiometric dating, medical imaging, and nuclear power. The half-life provides valuable information about the decay rate of radioactive isotopes and plays a significant role in the study of nuclear processes.
Looking for a more dynamic learning experience?
Explore our engaging video lessons and interactive animations that GoPhysics has to offer – your gateway to an immersive physics education!
GCSE Physics Tutorial - The Random Nature of Decay
In this tutorial, we will explore the concept that radioactive decay is random. Radioactive decay is a natural process in which unstable atomic nuclei transform into more stable configurations by emitting various types of radiation. Understanding that decay is a random process is crucial in nuclear physics and has practical implications in radiometric dating, medical imaging, and nuclear energy. Let's delve into why radioactive decay is considered a random phenomenon.
The Nature of Unstable Nuclei: Unstable atomic nuclei are characterised by having an excess of either protons or neutrons, making them energetically unstable. As a result, these nuclei are subject to radioactive decay to attain a more stable configuration.
The Decay Process: During radioactive decay, an unstable nucleus may undergo alpha decay ($ \alpha $), beta-minus decay ($ \beta^- $), beta-plus decay ($ \beta^+ $), gamma decay ($ \gamma $), electron capture ($ \text{EC} $), or positron emission ($ \text{β}^+ $). The type of decay and the time at which it occurs are unpredictable and random.
No External Influence: The decay of an unstable nucleus is not influenced by external factors such as temperature, pressure, or the presence of other particles. Each unstable nucleus has its own probability of decay, regardless of its surroundings.
Half-Life: The concept of half-life is used to describe the average time it takes for half of the radioactive nuclei in a sample to decay. However, it is important to note that the decay of individual nuclei is entirely random and not predictable.
Example of Random Decay: Imagine a sample of radioactive nuclei with a half-life of one hour. After the first hour, on average, half of the nuclei will have decayed. However, it is impossible to predict which individual nuclei will decay within that hour or when a specific nucleus will decay next.
Predicting Decay Times: Due to the random nature of decay, it is not possible to predict the exact time when an individual nucleus will decay. Nevertheless, we can make statistical predictions about the behaviour of a large group of radioactive nuclei.
Applications and Implications: The random nature of decay has practical applications in various fields. In radiometric dating, scientists can estimate the age of rocks and fossils by measuring the remaining amounts of certain radioactive isotopes. Additionally, in medical imaging and radiation therapy, understanding the random nature of decay is important for ensuring safe and effective practices.
In this tutorial, we have explored the concept that radioactive decay is a random process. Unstable atomic nuclei undergo decay in a probabilistic manner, independent of external influences. The random nature of decay is a fundamental aspect of nuclear physics and has significant applications in fields such as radiometric dating, medical imaging, and nuclear energy. Understanding this concept helps us interpret decay processes accurately and make informed decisions in various scientific and practical scenarios.
Looking for a more dynamic learning experience?
Explore our engaging video lessons and interactive animations that GoPhysics has to offer – your gateway to an immersive physics education!
GCSE Physics Tutorial - The Emission of Gamma Rays and Conservation of Mass and Charge
In this tutorial, we will explore the emission of gamma rays and how it does not cause the mass or charge of the nucleus to change. Gamma rays are high-energy electromagnetic waves emitted from the nucleus of an atom during gamma decay, a type of radioactive decay. Understanding this concept is important in nuclear physics to grasp the conservation of mass and charge during nuclear processes. Let's delve into why the emission of gamma rays does not affect the mass or charge of the nucleus.
Gamma Decay and Emission of Gamma Rays: Gamma decay is a process in which an excited atomic nucleus transitions to a lower energy state by releasing high-energy gamma rays. Unlike alpha and beta decay, gamma decay does not involve the emission of particles or cause the nucleus to change its identity. Instead, the nucleus emits a gamma ray (a photon of electromagnetic radiation) to release excess energy and reach a more stable state.
Mass Conservation: The emission of gamma rays during gamma decay does not alter the number of protons or neutrons in the nucleus. Therefore, the total mass of the nucleus remains unchanged before and after the emission of gamma rays. The mass number (A) of the parent nucleus is the same as the mass number of the daughter nucleus, indicating mass conservation.
Charge Conservation: Gamma decay does not involve the emission or absorption of charged particles (protons or electrons). As a result, the total charge of the nucleus remains unaffected during gamma decay. The parent nucleus and the daughter nucleus have the same number of protons (atomic number, Z), ensuring charge conservation.
Energy Conservation: Gamma decay is primarily driven by the need for the nucleus to reach a more stable energy state. The excess energy within the nucleus is released in the form of a gamma ray without altering the nucleus's identity or charge.
Representation in Decay Equations: Gamma decay is represented in decay equations as follows: \[ \text{Parent Nucleus} \rightarrow \text{Daughter Nucleus} + \gamma \]
In this tutorial, we have explored the emission of gamma rays during gamma decay and how it does not change the mass or charge of the nucleus. Gamma rays are high-energy electromagnetic waves emitted to release excess energy, allowing the nucleus to reach a more stable state. Mass and charge conservation are maintained throughout gamma decay, ensuring that the identity and charge of the nucleus remain the same. Understanding this concept is fundamental in nuclear physics and helps us comprehend the principles of energy conservation in radioactive decay processes.
Looking for a more dynamic learning experience?
Explore our engaging video lessons and interactive animations that GoPhysics has to offer – your gateway to an immersive physics education!
GCSE Physics Tutorial - Balancing Radioactive Decay Equations
Introduction: In this tutorial, we will learn how to balance radioactive decay equations. Radioactive decay equations represent the process by which unstable atomic nuclei transform into more stable configurations by emitting various types of radiation. Balancing these equations is crucial in nuclear physics to ensure that the total mass number (A) and atomic number (Z) are conserved on both sides of the equation. Balancing radioactive decay equations correctly allows us to accurately represent the decay process and understand the transformations of unstable nuclei. Let's delve into the key steps for balancing radioactive decay equations.
Steps to Balance Radioactive Decay Equations:
Identify the Decay Mode: The first step is to identify the decay mode from the decay equation. Common decay modes include alpha decay ($ \alpha $), beta-minus decay ($ \beta^- $), beta-plus decay ($ \beta^+ $), gamma decay ($ \gamma $), electron capture ($ \text{EC} $), and positron emission ($ \text{β}^+ $).
Determine the Daughter Nucleus: Next, determine the daughter nucleus that results from the decay process. The daughter nucleus is the resulting nucleus after the decay of the parent nucleus. It may have a different atomic number (Z) and mass number (A) compared to the parent nucleus.
Write the Decay Equation: Write the initial decay equation by placing the parent nucleus on the left-hand side and the daughter nucleus and emitted particle or radiation on the right-hand side. Include the symbols for the respective decay mode and the emitted particles.
Mass Number (A) Conservation: Ensure that the total mass number (A) is conserved on both sides of the equation. The sum of A on the left-hand side (parent nucleus) should be equal to the sum of A on the right-hand side (daughter nucleus and emitted particles).
Atomic Number (Z) Conservation: Ensure that the total atomic number (Z) is conserved on both sides of the equation. The sum of Z on the left-hand side (parent nucleus) should be equal to the sum of Z on the right-hand side (daughter nucleus and emitted particles).
Balance by Adjusting the Emitted Particles: If the equation is not balanced, adjust the number of emitted particles on the right-hand side to balance the equation. For example, if the equation involves the emission of two beta particles, make sure to include two beta particles on the right-hand side.
Verify the Balanced Equation: After making adjustments, verify that the equation is balanced by checking that the total mass number (A) and atomic number (Z) are equal on both sides.
Example Equations:
a. Alpha Decay:
b. Beta-Minus Decay:
c. Beta-Plus Decay:
d. Gamma Decay:
e. Electron Capture:
Conclusion: In this tutorial, we have learned how to balance radioactive decay equations correctly. By identifying the decay mode, determining the daughter nucleus, and ensuring the conservation of mass number (A) and atomic number (Z), we can accurately represent the decay process. Balancing these equations is essential in nuclear physics to understand the transformations of unstable atomic nuclei and their applications in radiometric dating, medical imaging, and nuclear energy.
Looking for a more dynamic learning experience?
Explore our engaging video lessons and interactive animations that GoPhysics has to offer – your gateway to an immersive physics education!
GCSE Physics Tutorial - Predicting Types of Emission from Decay Equations
In this tutorial, we will learn how to predict the types of emission from radioactive decay equations. Radioactive decay is a natural process in which unstable atomic nuclei transform into more stable configurations by emitting various types of radiation. Understanding how to predict the types of emission is crucial in nuclear physics and has practical applications in radiometric dating, medical imaging, and nuclear energy. Let's delve into the key steps for predicting the types of emission from decay equations.
Identify the Decay Mode: The first step in predicting the type of emission is to identify the decay mode from the decay equation. Common decay modes include alpha decay ($ \alpha $), beta-minus decay ($ \beta^- $), beta-plus decay ($ \beta^+ $), gamma decay ($ \gamma $), electron capture ($ \text{EC} $), and positron emission ($ \text{β}^+ $).
Determine the Daughter Nucleus: Next, determine the daughter nucleus that results from the decay process. The daughter nucleus is the resulting nucleus after the decay of the parent nucleus. It may have a different atomic number (Z) and mass number (A) compared to the parent nucleus.
Identify the Emitted Particle or Radiation: Based on the decay mode, identify the emitted particle or radiation in the decay equation. Each decay mode involves the emission of specific particles or radiation:
a. Alpha Decay ($ \alpha $): In alpha decay, an alpha particle ($ ^4_2\text{He} $) is emitted from the parent nucleus. b. Beta-Minus Decay ($ \beta^- $): In beta-minus decay, a beta particle ($ ^0_{-1}\text{e} $) is emitted from the parent nucleus. c. Beta-Plus Decay ( ${\beta^+ }$ ): In beta-plus decay, a positron ($ ^0_{+1}\text{e} $) is emitted from the parent nucleus. d. Gamma Decay ($ \gamma $): In gamma decay, a gamma ray ($ \gamma $) is emitted from the parent nucleus. e. Electron Capture ($\text{EC} $): In electron capture, an electron is captured by the nucleus, resulting in the emission of a neutrino ($ \nu $) and a photon (X-ray or gamma ray).
Verify Mass and Atomic Number Conservation: Ensure that the decay equation satisfies the conservation of mass number (A) and atomic number (Z) on both sides of the equation. The sum of A and Z of the parent and daughter nuclei must be equal.
Example Equations:
a. Alpha Decay:
b. Beta-Minus Decay:
c. Beta-Plus Decay:
d. Gamma Decay:
e. Electron Capture:
In this tutorial, we have learned how to predict the types of emission from radioactive decay equations. By identifying the decay mode and determining the daughter nucleus, we can determine the emitted particle or radiation in the decay equation. Alpha decay emits an alpha particle ($ \alpha $), beta-minus decay emits a beta particle ($\beta^- $), beta-plus decay emits a positron ($\beta^+ $), gamma decay emits a gamma ray ($ \gamma $), and electron capture emits a neutrino ($ \nu $) and a photon (X-ray or gamma ray). Understanding how to predict the types of emission is essential for interpreting decay processes in nuclear physics and their applications in various scientific fields.
Looking for a more dynamic learning experience?
Explore our engaging video lessons and interactive animations that GoPhysics has to offer – your gateway to an immersive physics education!
GCSE Physics Tutorial - Recognising Qualities in Radioactive Decay Equations
In this tutorial, we will explore the qualities present in radioactive decay equations. Radioactive decay equations represent the process by which unstable atomic nuclei transform into more stable configurations by emitting various types of radiation. Understanding the qualities of these equations is essential in nuclear physics and has practical applications in radiometric dating, medical imaging, and nuclear energy. Let's delve into the key qualities found in radioactive decay equations.
General Radioactive Decay Equation: The general form of a radioactive decay equation is written as follows: $$\text{Parent Nucleus} \rightarrow \text{Daughter Nucleus} + \text{Radiation} $$
Representation of Decay Mode: The decay mode is indicated by the type of radiation emitted in the equation. Common decay modes include alpha decay (${( \alpha )}$), beta-minus decay (${ \beta^- }$), beta-plus decay (${ \beta^+ }$), gamma decay (${ \gamma }$), electron capture (${ \text{EC} }$), and positron emission (${ \text{β}^+ }$).
Parent and Daughter Nuclei: The parent nucleus is the initial unstable radioactive isotope that undergoes decay. The daughter nucleus is the resulting nucleus after the decay process. The daughter nucleus may have a different atomic number (Z) and mass number (A) compared to the parent nucleus.
Mass Number Conservation: In a decay equation, the sum of the mass numbers (A) of the parent and daughter nuclei on both sides must be equal.
Atomic Number Conservation: The total atomic number (Z) of the parent and daughter nuclei on both sides of the equation must also be equal.
Emission of Radiation: The type of radiation emitted during decay is indicated in the equation. For example, alpha decay involves the emission of an alpha particle (${ \alpha }$), beta-minus decay emits a beta particle ($ {\beta^- }$), and gamma decay releases a gamma ray (${ \gamma }$).
Change in Atomic Number: In some decay modes, the atomic number (Z) changes, leading to a different element in the daughter nucleus. For example, beta-minus decay increases the atomic number by one, while beta-plus decay decreases the atomic number by one.
Change in Mass Number: In alpha decay, the mass number (A) of the parent nucleus decreases by four units, while the atomic number (Z) decreases by two.
Half-Life Representation: Decay equations do not explicitly include half-life values. Half-life is a separate property associated with each radioactive isotope, indicating the time it takes for half of the initial quantity of radioactive nuclei to decay.
Example Equations:
a. Alpha Decay:
b. Beta-Minus Decay:
c. Gamma Decay:
In this tutorial, we have explored the qualities found in radioactive decay equations. These equations represent the process of radioactive decay and are essential in understanding the transformations of unstable atomic nuclei into more stable configurations. The qualities include representation of decay mode, conservation of mass and atomic numbers, and the emission of radiation. Recognising these qualities is fundamental in nuclear physics and has diverse applications in radiometric dating, medical imaging, and nuclear energy.
Looking for a more dynamic learning experience?
Explore our engaging video lessons and interactive animations that GoPhysics has to offer – your gateway to an immersive physics education!
GCSE Physics Tutorial - Applying Decay Properties in Uses of Radiation
In this tutorial, we will explore how decay properties of radioactive substances are applied in various uses of radiation. Understanding decay properties is crucial in determining the appropriate sources of radiation for specific applications. Different radioactive isotopes have unique decay characteristics that make them suitable for various purposes, including medical imaging, radiometric dating, and industrial applications. Let's delve into how decay properties are utilised to evaluate the best sources of radiation in different situations.
Medical Imaging and Radiotherapy: a. Gamma Emitters: Gamma rays have high penetrating power and are commonly used in medical imaging techniques like gamma camera imaging and positron emission tomography (PET). They can also be used in radiotherapy to treat cancerous tumors.
b. Beta Emitters: Some beta emitters like technetium-99m (99mTc) are used in nuclear medicine for diagnostic purposes. They emit beta particles, which can be detected by imaging devices to visualise specific body functions or organs.
c. Alpha Emitters: Alpha emitters are generally not used in medical imaging due to their low penetrating power. However, some targeted alpha therapies are being explored for treating certain types of cancer.
Radiometric Dating: a. Carbon-14: Carbon-14 dating is used to determine the age of organic materials. It is based on the decay of carbon-14, a beta emitter, into nitrogen-14. The half-life of carbon-14 is about 5,730 years, making it suitable for dating materials up to around 50,000 years old.
b. Uranium-Series Dating: Uranium isotopes, like uranium-238 and uranium-235, decay through a series of isotopes until they reach stable lead isotopes. This decay series is used to date rocks and minerals that are millions of years old.
Industrial Applications: a. Gamma Sources: Gamma emitters like cobalt-60 and iridium-192 are used in industrial radiography to inspect welds and structures for defects. They can also be used in gauging applications to measure the density and thickness of materials.
b. Neutron Sources: Neutron sources, like americium-beryllium and californium-252, are used in certain industrial applications, including neutron radiography and activation analysis.
Evaluating the Best Sources of Radiation: When choosing the best source of radiation for a specific application, several factors need to be considered:
a. Half-Life: The half-life of the radioactive isotope should match the time scale of the application. For short-term imaging, short-lived isotopes are preferred, while long-lived isotopes are used in long-term industrial applications.
b. Penetration: The penetrating power of the emitted radiation should be appropriate for the material being analysed or treated.
c. Safety: Safety considerations, such as shielding and handling procedures, are critical when dealing with radioactive materials.
d. Specific Decay Mode: The decay mode of the isotope should be suitable for the desired application. For instance, beta emitters are preferred for medical imaging, while gamma emitters are used in industrial radiography.
In this tutorial, we have explored how decay properties of radioactive substances are applied in various uses of radiation. Different decay modes and half-lives of isotopes make them suitable for specific applications in medical imaging, radiometric dating, and industrial uses. When evaluating the best sources of radiation for a given situation, factors like half-life, penetration, safety, and specific decay mode must be considered to ensure successful and safe applications.
Looking for a more dynamic learning experience?
Explore our engaging video lessons and interactive animations that GoPhysics has to offer – your gateway to an immersive physics education!
GCSE Physics Tutorial - Decay Property Qualities
In this tutorial, we will describe the key decay property qualities associated with radioactive substances. Radioactive decay is a natural process in which unstable atomic nuclei transform into more stable configurations by emitting various types of radiation. Understanding the decay property qualities is essential in nuclear physics and has practical applications in radiometric dating, medical imaging, and nuclear energy. Let's delve into the important characteristics of radioactive decay.
Decay Constant (λ): The decay constant, denoted by the symbol "λ," is a fundamental property of a radioactive substance. It represents the probability of a single radioactive decay occurring in a given unit of time. The higher the decay constant, the faster the rate of decay and vice versa. The decay constant is inversely related to the half-life of the substance.
Half-Life (T½): The half-life of a radioactive substance is the time it takes for half of the initial quantity of radioactive nuclei to decay. It is a characteristic property unique to each radioactive isotope. The half-life determines the rate at which a radioactive substance loses its radioactivity. Substances with shorter half-lives decay faster, while those with longer half-lives decay more slowly.
Activity (A): Activity is a measure of the rate of radioactive decay of a substance. It represents the number of radioactive decays that occur per unit of time within a given radioactive source. The activity is directly proportional to the decay constant (λ) and the number of radioactive nuclei present in the sample.
Decay Mode: The decay mode refers to the type of radiation emitted during radioactive decay. The main decay modes are alpha decay, beta decay (including beta-minus and beta-plus decay), gamma decay, electron capture, and positron emission. Each decay mode involves the emission of specific particles or radiation from the unstable nucleus.
Radiation Type and Penetrating Power: The emitted radiation during decay can be of different types, including alpha particles (low penetrating power), beta particles (moderate penetrating power), and gamma rays (high penetrating power). The penetrating power of the emitted radiation depends on its energy and type.
Stability: The stability of a nucleus depends on the balance between the number of protons and neutrons it contains. Stable nuclei have an optimal neutron-to-proton ratio, while unstable nuclei have an imbalance, leading to radioactive decay.
Decay Series: Some radioactive isotopes decay into other unstable isotopes, which further undergo decay in a series of steps until a stable isotope is reached. This series of decays is called a decay series. Uranium and thorium decay series are well-known examples.
In this tutorial, we have described the key decay property qualities associated with radioactive substances. Decay constant (λ), half-life (T½), and activity (A) are essential properties that govern the rate of radioactive decay. Understanding the decay mode, radiation type, penetrating power, stability, and decay series is crucial in nuclear physics and has diverse applications in various scientific and practical fields, including radiometric dating, medical imaging, and nuclear energy.
Looking for a more dynamic learning experience?
Explore our engaging video lessons and interactive animations that GoPhysics has to offer – your gateway to an immersive physics education!
GCSE Physics Tutorial - Types of Radioactive Decay
In this tutorial, we will explore the different types of radioactive decay that occur in unstable atomic nuclei. Radioactive decay is a natural process through which unstable nuclei transform into more stable configurations by emitting various types of radiation. Understanding the different types of decay is crucial in nuclear physics and has practical applications in radiometric dating, medical imaging, and nuclear energy. Let's delve into the key types of radioactive decay.
Alpha Decay: Alpha decay involves the emission of alpha particles from the nucleus of a radioactive atom. An alpha particle consists of two protons and two neutrons, which is the same as a helium nucleus. The emission of an alpha particle reduces the atomic number (Z) of the parent nucleus by two and its mass number (A) by four.
Example: Uranium-238 (238U) undergoing alpha decay becomes Thorium-234 (234Th) with the emission of an alpha particle (4He).
Beta Decay: Beta decay occurs when a nucleus has either too many protons or too few neutrons to be stable. There are two types of beta decay:
a. Beta-Minus (β-): In beta-minus decay, a neutron in the nucleus is converted into a proton, and an electron (beta-minus particle) is emitted from the nucleus. This increases the atomic number (Z) of the parent nucleus by one but leaves the mass number (A) unchanged.
Example: Carbon-14 (14C) undergoing beta-minus decay becomes Nitrogen-14 (14N) with the emission of an electron (e-).
b. Beta-Plus (β+): In beta-plus decay, a proton in the nucleus is converted into a neutron, and a positron (beta-plus particle) is emitted from the nucleus. This decreases the atomic number (Z) of the parent nucleus by one but leaves the mass number (A) unchanged.
Example: Fluorine-18 (18F) undergoing beta-plus decay becomes Oxygen-18 (18O) with the emission of a positron (e+).
Gamma Decay: Gamma decay involves the emission of high-energy gamma rays from an excited atomic nucleus. Unlike alpha and beta decay, gamma decay does not alter the atomic number (Z) or the mass number (A) of the parent nucleus. Gamma rays are electromagnetic radiation and do not consist of particles like alpha and beta particles.
Example: Technetium-99m (99mTc) undergoing gamma decay transitions to Technetium-99 (99Tc) without changing its atomic number or mass number.
Other Types of Decay: There are other less common types of radioactive decay, such as: a. Electron Capture (EC): A proton captures an electron from the inner electron shell, converting into a neutron. b. Positron Emission (β+): A proton converts into a neutron, emitting a positron. c. Spontaneous Fission: Heavy atomic nuclei split into two smaller nuclei.
In this tutorial, we have explored the types of radioactive decay that occur in unstable atomic nuclei. Alpha decay involves the emission of alpha particles, beta decay includes beta-minus and beta-plus emissions, gamma decay involves the emission of gamma rays, and there are other less common decay modes. Understanding these types of decay is fundamental in nuclear physics and has wide-ranging applications in fields such as radiometric dating, medical imaging, and nuclear energy.
Looking for a more dynamic learning experience?
Explore our engaging video lessons and interactive animations that GoPhysics has to offer – your gateway to an immersive physics education!
GCSE Physics Tutorial - Define Count Rate
In this tutorial, we will explore the concept of count rate in the context of radiation detection and measurement. Count rate is a fundamental term used to describe the number of radiation events detected per unit of time by a radiation detector. Let's delve into the definition and significance of count rate in the study of radiation and nuclear physics.
Definition of Count Rate: Count rate is the number of radiation events or radioactive decays detected by a radiation detector per unit of time. It provides a measure of the intensity of radiation emitted by a radioactive source or encountered in a specific environment.
Units of Count Rate: The standard unit of count rate is counts per second (cps) or counts per minute (cpm). Count rate is a measure of the rate at which radiation is detected and is directly related to the activity of the radiation source.
Radiation Detectors: Various radiation detectors, such as Geiger-Muller counters, scintillation detectors, and proportional counters, are used to measure count rates. These detectors can detect different types of radiation, including alpha particles, beta particles, gamma rays, and X-rays.
Importance of Count Rate: Count rate is crucial for various applications, including nuclear physics research, radiological protection, environmental monitoring, and medical imaging. It allows scientists and professionals to assess the level of radiation present and the potential risks associated with radioactive materials or sources.
Factors Affecting Count Rate: Several factors can affect the count rate measured by a radiation detector, including: a. Activity of the Source: Higher radioactive activity results in a higher count rate due to an increased number of radioactive decays per unit of time. b. Distance from the Source: As the distance from the radioactive source increases, the count rate decreases because fewer radiation events reach the detector. c. Shielding: Shielding materials can attenuate or block radiation, leading to a reduced count rate. d. Background Radiation: Background radiation from natural or man-made sources contributes to the overall count rate in a given environment.
Data Collection and Analysis: When using a radiation detector, the count rate is continuously or periodically recorded over a specific time interval. This data is then analysed to determine the radiation level, activity of the source, or assess the presence of any abnormal radiation levels.
In this tutorial, we have defined count rate as the number of radiation events detected per unit of time by a radiation detector. Count rate is an essential measure in radiation detection and is used to quantify the intensity of radiation emitted by a radioactive source or encountered in a specific environment. It is expressed in units of counts per second (cps) or counts per minute (cpm) and plays a crucial role in various applications, including nuclear physics research, radiation safety, and medical imaging. Understanding count rate is fundamental for anyone working with radiation detectors or studying nuclear and radiation physics.
Looking for a more dynamic learning experience?
Explore our engaging video lessons and interactive animations that GoPhysics has to offer – your gateway to an immersive physics education!
GCSE Physics Tutorial - Unit of Activity: The Becquerel (Bq)
In this tutorial, we will explore the unit of activity used to measure the rate of radioactive decay, known as the Becquerel (Bq). The Becquerel is an essential unit in nuclear physics, providing a standardised way to quantify the intensity of radiation emitted by a radioactive substance. Let's delve into the definition and significance of the Becquerel as the unit of activity.
Definition of Activity: Activity is a measure of the rate at which a radioactive substance undergoes decay. It represents the number of radioactive decays that occur per unit of time within a given radioactive source.
The Becquerel (Bq): The Becquerel, denoted by the symbol "Bq," is the SI unit of activity. One Becquerel is equal to one radioactive decay per second.
Relationship to Second: The unit "per second" in the definition of the Becquerel emphasises that activity is a rate measurement. It indicates that the number of radioactive decays is counted within a time interval of one second.
Named After Henri Becquerel: The Becquerel is named after Henri Becquerel, a French physicist who, in 1896, discovered radioactivity while working with uranium salts. His discovery laid the foundation for the study of radioactivity and the development of nuclear physics.
Alternative Units: In the past, activity was expressed using non-SI units such as the Curie (Ci) and the Rutherford (rd). However, the International System of Units (SI) introduced the Becquerel as the standard unit for activity to promote international uniformity in measurements.
Conversion Factors: To convert between the Becquerel and older non-SI units: a. 1 Becquerel (Bq) = 1 radioactive decay per second. b. 1 Curie (Ci) = 3.7 x 10^10 Bq. c. 1 Rutherford (rd) = 10^6 Bq.
Importance of the Becquerel: The Becquerel is a fundamental unit in nuclear physics and is widely used in various scientific and practical applications. It is essential in fields such as nuclear medicine, radiography, environmental monitoring, and industrial applications involving radioactive materials.
In this tutorial, we have learned that the unit of activity used to measure the rate of radioactive decay is the Becquerel (Bq). One Becquerel represents one radioactive decay per second. The Becquerel is an SI unit, providing a standardised and internationally recognised method to quantify the intensity of radiation emitted by a radioactive substance. Its introduction has led to greater uniformity in scientific measurements and has been instrumental in various fields, including nuclear medicine and radiography. Understanding the Becquerel is fundamental for anyone working with radioactive materials or studying nuclear physics.
Looking for a more dynamic learning experience?
Explore our engaging video lessons and interactive animations that GoPhysics has to offer – your gateway to an immersive physics education!
GCSE Physics Tutorial - Define Activity
In this tutorial, we will explore the concept of activity in the context of nuclear physics. Activity is a fundamental measure used to quantify the rate at which a radioactive substance undergoes decay. It provides crucial information about the intensity of radiation emitted by a radioactive source. Let's delve into the definition and key aspects of activity.
Definition of Activity: Activity, denoted by the symbol "A," is a measure of the rate of radioactive decay of a substance. It represents the number of radioactive decays that occur per unit of time within a given radioactive source.
Units of Activity: The standard unit of activity in the International System of Units (SI) is the Becquerel (Bq). One Becquerel is equal to one radioactive decay per second (1 Bq = 1 decay/s).
Curie and Rutherford: In older non-SI units, the Curie (Ci) and the Rutherford (rd) were used to express activity: a. Curie (Ci): One Curie represents 3.7 x 10^10 decays per second (1 Ci = 3.7 x 10^10 Bq). b. Rutherford (rd): One Rutherford represents 10^6 decays per second (1 rd = 10^6 Bq).
Activity and Half-Life: The activity of a radioactive substance is directly related to its half-life. As the half-life decreases, the activity increases because more radioactive decays occur in a given time period.
Measuring Activity: Activity can be measured using a radiation detector, such as a Geiger-Muller counter or a scintillation detector. These instruments detect and count the radioactive decays emitted by a radioactive source.
Decay Constant: The rate of radioactive decay is governed by the decay constant (λ). The decay constant represents the probability of a single radioactive decay occurring in a given unit of time. It is related to the half-life (T½) by the equation: λ = ln(2) / T½.
Calculating Activity: The activity (A) of a radioactive substance can be calculated using the formula: A = λ * N, where N is the number of radioactive nuclei present in the sample.
Importance of Activity: Activity is a crucial parameter in understanding and managing radioactive materials. It is used in various applications, including nuclear medicine, radiography, and industrial applications.
In this tutorial, we have defined activity as the rate of radioactive decay of a substance. It is a measure of the number of radioactive decays that occur per unit of time within a radioactive source. Activity is quantified using the SI unit Becquerel (Bq) and provides essential information about the intensity of radiation emitted by a radioactive material. The activity of a substance is directly related to its half-life and decay constant. Understanding activity is fundamental in nuclear physics and plays a vital role in various practical applications, such as nuclear medicine and industrial uses of radioactive materials.
Looking for a more dynamic learning experience?
Explore our engaging video lessons and interactive animations that GoPhysics has to offer – your gateway to an immersive physics education!
GCSE Physics Tutorial - Radioactive Decay: The Random Process of Nuclei Becoming More Stable
In this tutorial, we will explore the concept of radioactive decay, a random process through which atomic nuclei release radiation as they transform to become more stable. This phenomenon occurs in unstable nuclei, leading to the emission of various types of radiation. Let's delve into the key features of radioactive decay and its role in stabilising unstable atomic nuclei.
Radioactive Decay: Radioactive decay is a natural process by which certain atomic nuclei spontaneously undergo transformations to reach a more stable state. This process allows unstable nuclei to release excess energy and achieve a more balanced configuration of protons and neutrons.
Unstable Nuclei and Stability: Unstable nuclei have an excess of energy due to an imbalance of protons and neutrons. To attain stability, these nuclei undergo radioactive decay, a process that reduces their energy level and brings them closer to a more balanced configuration.
Types of Radiation: During radioactive decay, unstable nuclei emit different types of radiation: a. Alpha Particles (α): Alpha decay involves the emission of alpha particles, which are helium nuclei composed of two protons and two neutrons. b. Beta Particles (β): Beta decay occurs when a neutron is transformed into a proton or vice versa, leading to the emission of beta particles (electrons or positrons). c. Gamma Rays (γ): Gamma decay involves the emission of high-energy gamma rays, which are a form of electromagnetic radiation.
Random Process: Radioactive decay is a random process, meaning it cannot be predicted when a specific nucleus will undergo decay. Each unstable nucleus has its own characteristic decay rate, expressed as a half-life.
Half-Life: The half-life of a radioactive substance is the time it takes for half of the initial quantity of radioactive nuclei to decay. Different radioactive isotopes have different half-lives, ranging from fractions of a second to billions of years.
Importance of Half-Life: The concept of half-life is crucial for understanding the rate of decay of a radioactive substance. It helps determine how quickly a sample of radioactive material will lose its radioactivity over time.
Applications of Radioactive Decay: Radioactive decay plays a significant role in various scientific and practical applications, including: a. Radiometric Dating: The half-life of certain isotopes can be used to determine the age of rocks and fossils. b. Medical Imaging and Treatment: Radioactive isotopes are used in medical imaging (e.g., PET scans) and radiation therapy to treat diseases like cancer. c. Nuclear Energy: Controlled nuclear decay is harnessed in nuclear power plants to generate electricity.
In this tutorial, we have explored the concept of radioactive decay, a random process through which unstable atomic nuclei emit radiation as they become more stable. Radioactive decay leads to the transformation of unstable nuclei into more balanced configurations, releasing excess energy in the form of alpha particles, beta particles, and gamma rays. The half-life of a radioactive substance plays a crucial role in determining the rate of decay, making it essential for various scientific applications. Understanding radioactive decay is fundamental in nuclear physics and has wide-ranging practical applications in radiometric dating, medical imaging, and nuclear energy production.
Looking for a more dynamic learning experience?
Explore our engaging video lessons and interactive animations that GoPhysics has to offer – your gateway to an immersive physics education!
GCSE Physics Tutorial - Unstable Atomic Nuclei
In this tutorial, we will explore the concept of unstable atomic nuclei. Atoms consist of a positively charged nucleus surrounded by negatively charged electrons. While many nuclei are stable and exist indefinitely, some atomic nuclei are inherently unstable and undergo spontaneous changes, releasing energy in the process. Let's delve into the key features of unstable atomic nuclei and understand the implications of their instability.
Stability of Atomic Nuclei: The stability of an atomic nucleus is determined by the balance between the forces that hold protons and neutrons together and the forces that cause them to repel due to their like charges.
Stable Nuclei: Nuclei with a balanced number of protons and neutrons tend to be stable. These stable nuclei remain unchanged over time and do not spontaneously decay.
Unstable Nuclei: Unstable nuclei have an imbalance of protons and neutrons, making them less energetically favorable. As a result, these nuclei tend to undergo spontaneous decay, transforming into other nuclei and emitting various forms of radiation.
Radioactive Decay: The process by which an unstable atomic nucleus spontaneously transforms into a more stable nucleus is known as radioactive decay. During this process, the nucleus releases energy in the form of radiation.
Types of Radioactive Decay: There are several types of radioactive decay, including: a. Alpha Decay: In alpha decay, an alpha particle (consisting of two protons and two neutrons) is emitted from the nucleus. b. Beta Decay: In beta decay, a neutron is converted into a proton or vice versa, and a beta particle (an electron or a positron) is emitted from the nucleus. c. Gamma Decay: Gamma decay involves the emission of a high-energy gamma ray, which is a form of electromagnetic radiation. d. Other Forms of Decay: Some unstable nuclei undergo other types of decay, such as positron emission, electron capture, or spontaneous fission.
Half-Life: The half-life of a radioactive substance is the time it takes for half of the initial amount of radioactive nuclei to decay. Different radioactive isotopes have different half-lives, ranging from fractions of a second to billions of years.
Importance of Unstable Nuclei: Unstable atomic nuclei are of significant interest to scientists and researchers. The study of unstable nuclei and their decay processes is crucial for understanding nuclear physics, radiometric dating, medical imaging, and nuclear energy applications.
In this tutorial, we have explored the concept of unstable atomic nuclei. Some atomic nuclei are inherently unstable due to an imbalance of protons and neutrons, leading to spontaneous radioactive decay. This decay process releases energy in the form of radiation. Understanding unstable nuclei and their behaviour is essential for various scientific applications, including radiometric dating, medical treatments, and nuclear energy generation. The study of unstable nuclei continues to be a fascinating and critical field in modern physics.
Looking for a more dynamic learning experience?
Explore our engaging video lessons and interactive animations that GoPhysics has to offer – your gateway to an immersive physics education!
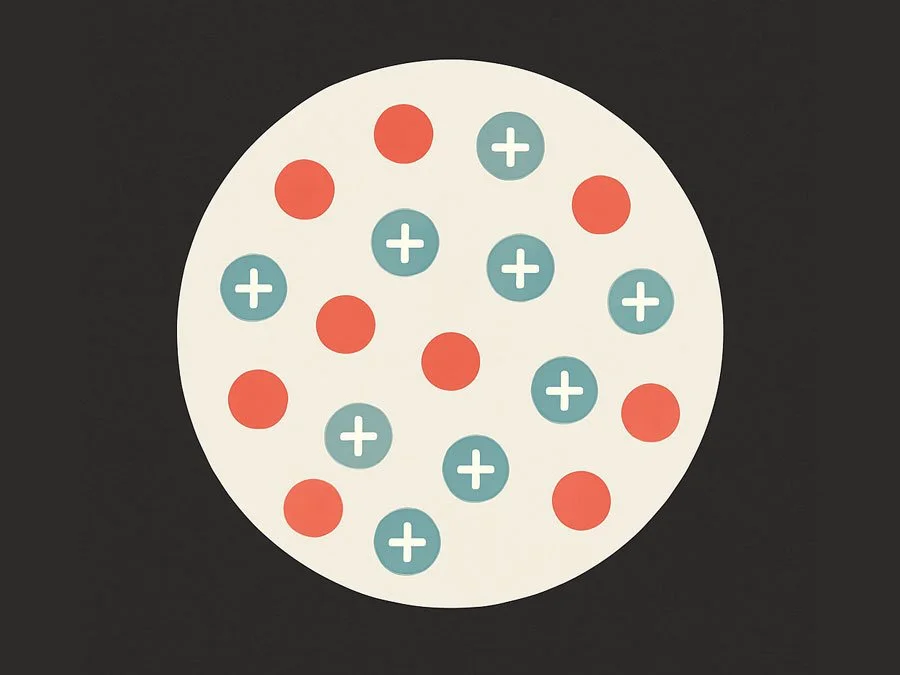
Difference Between the Plum Pudding Model and the Nuclear Model of the Atom
In this tutorial, we’ll explore the key differences between two important atomic models: the Plum Pudding model and the Nuclear model. Both played a major role in the development of atomic theory, but they differ in how they explain the atom’s structure and distribution of charge.
The Plum Pudding Model (J.J. Thomson, late 1800s)
This was one of the earliest models of the atom. According to Thomson:
Structure: The atom was thought to be a soft, positively charged sphere.
Electrons: Electrons were scattered throughout this sphere—like raisins in a plum pudding.
Charge: The positive and negative charges were evenly spread, so the atom was overall neutral.
The Nuclear Model (Ernest Rutherford, early 1900s)
Rutherford’s model was developed after his gold foil experiment, which changed how scientists viewed the atom:
Nucleus: Most of the atom’s mass and positive charge is packed into a small, dense core called the nucleus.
Electrons: Electrons orbit around the nucleus, similar to planets orbiting the sun.
Charge: The positive charge of the nucleus is balanced by the negative electrons—so the atom remains neutral overall.
Key Differences
1. Charge Distribution
Plum Pudding: Charge is spread evenly throughout the atom.
Nuclear Model: Positive charge is concentrated in the centre (nucleus), with electrons surrounding it.
2. Presence of a Nucleus
Plum Pudding: No nucleus—just a uniform sphere of charge.
Nuclear Model: A central nucleus holds most of the atom’s mass and positive charge.
3. Experimental Support
Plum Pudding: Based on theory, with no strong experimental evidence.
Nuclear Model: Supported by Rutherford’s gold foil experiment, which showed that some alpha particles bounced back—impossible if charge was spread evenly.
Summary
The Plum Pudding model suggested that atoms were soft spheres with evenly spread charge. The Nuclear model introduced the idea of a dense, central nucleus, with electrons orbiting around it — an idea backed by experimental evidence. This shift marked a turning point in atomic theory and laid the foundation for the more advanced models we use today.
Related Posts:
The Atomic Model Before the Discovery of the Electron
The Discovery of the Electron and the Plum Pudding Model of the Atom
GCSE Physics Tutorial - Scattering Experiments and the Changing Atomic Model
Introduction: In this tutorial, we will explore how new evidence from scattering experiments led to significant changes in the atomic model. Scientists conducted these experiments to investigate the structure of the atom and the behaviour of subatomic particles. The results of these experiments challenged existing atomic models and paved the way for a deeper understanding of atomic structure. Let's delve into the key experiments and the impact they had on shaping the atomic model.
Background: Before the advent of modern atomic models, the prevailing model was the Plum Pudding model, proposed by J.J. Thomson. According to this model, the atom was considered a positively charged sphere with electrons dispersed throughout.
The Gold Foil Experiment: Ernest Rutherford and his colleagues conducted the gold foil experiment, also known as the alpha particle scattering experiment. In this experiment, they directed alpha particles (positively charged particles) at a thin sheet of gold foil.
Unexpected Results: Contrary to expectations based on the Plum Pudding model, Rutherford observed that some alpha particles were deflected at large angles, and a few even bounced straight back. This indicated that most of the atom's mass and positive charge were concentrated in a tiny, dense region at the centre, which Rutherford named the nucleus.
Rutherford's Nuclear Model: Based on the experimental results, Rutherford proposed a new atomic model called the "nuclear model" or "planetary model." According to this model: a. The nucleus: The majority of the atom's mass and positive charge are concentrated in the nucleus, which is tiny compared to the overall size of the atom. b. Electrons: Electrons, being much lighter and negatively charged, revolve around the nucleus at significant distances.
The Discovery of Protons: Subsequent experiments by other scientists led to the discovery of protons, positively charged particles within the nucleus. This discovery further supported the nuclear model and provided evidence that the positive charge of the nucleus could be subdivided into smaller particles.
The Discovery of Neutrons: Experiments by James Chadwick revealed the existence of neutrons, neutral particles also present in the nucleus. This discovery completed the understanding of atomic nuclei as composed of protons and neutrons, with electrons orbiting around the nucleus.
Quantum Mechanics and Modern Atomic Models: The development of quantum mechanics in the 20th century provided a deeper understanding of the behaviour of subatomic particles and their interaction within atoms. Quantum mechanics laid the foundation for the modern atomic model, which incorporates wave-particle duality and the concept of atomic orbitals.
Conclusion: In this tutorial, we have explored how new evidence from scattering experiments led to significant changes in the atomic model. Rutherford's gold foil experiment revealed the presence of a positively charged nucleus at the centre of the atom, leading to the nuclear model. The discovery of protons and neutrons within the nucleus further refined the model and completed the understanding of atomic nuclei. Subsequent developments in quantum mechanics paved the way for the modern atomic model, which considers the dual nature of particles and the behaviour of electrons within atomic orbitals. These scattering experiments and the changing atomic model have revolutionised our understanding of atomic structure, laying the foundation for further advancements in nuclear physics and quantum mechanics.
Looking for a more dynamic learning experience?
Explore our engaging video lessons and interactive animations that GoPhysics has to offer – your gateway to an immersive physics education!
GCSE Physics Tutorial - Discovery of Neutrons by James Chadwick
In this tutorial, we will explore the experimental work of James Chadwick, which provided crucial evidence for the existence of neutrons within the atomic nucleus. Chadwick's groundbreaking discovery came about 20 years after the concept of the atomic nucleus was accepted in scientific circles. Let's delve into the key experiments and insights that led to the identification of neutrons as another essential constituent of the atomic nucleus.
Background: Following Ernest Rutherford's discovery of the atomic nucleus in the early 20th century, scientists sought to understand the nature of the positively charged protons within the nucleus. However, there was a discrepancy between the mass of the nucleus, as determined by the total number of protons, and its observed mass from experiments.
The Mass Defect: Experiments showed that the mass of a nucleus was slightly less than the sum of the masses of its individual protons and electrons. This discrepancy became known as the "mass defect."
Chadwick's Experiments: In the early 1930s, James Chadwick conducted experiments to investigate the origin of the mass defect and provide a more complete understanding of the atomic nucleus.
The Discovery of Neutrons: Chadwick's key experiments involved bombarding beryllium with alpha particles. He observed that the alpha particles were scattered, and additional radiation was produced. This additional radiation was neutral and had a mass slightly larger than a proton, consistent with the mass defect.
Neutron Emission: Chadwick concluded that the additional radiation emitted during the beryllium-alpha particle collisions consisted of neutral particles with a mass similar to that of a proton. He named these neutral particles "neutrons."
Neutrons and the Mass Defect: Chadwick's discovery of neutrons explained the mass defect observed in nuclear experiments. The neutrons accounted for the missing mass and played a crucial role in balancing the positive charges of protons in the nucleus.
Electrical Neutrality of Neutrons: Neutrons carry no electrical charge, making them electrically neutral. Unlike protons and electrons, which carry positive and negative charges, respectively, neutrons have no net charge.
Significance of Chadwick's Discovery: Chadwick's discovery of neutrons solidified the understanding of atomic nuclei as composed of protons and neutrons, with electrons orbiting around the nucleus. This discovery further enhanced the nuclear model of the atom, providing a more comprehensive picture of atomic structure.
Later Contributions: Chadwick's discovery of neutrons opened the door for further research into nuclear physics and led to the development of nuclear energy and modern particle physics.
In this tutorial, we have explored the experimental work of James Chadwick, which provided crucial evidence for the existence of neutrons within the atomic nucleus. Chadwick's discovery, about 20 years after the acceptance of the atomic nucleus, filled the gap in understanding the mass defect and unveiled the presence of neutral particles, the neutrons, in the nucleus. This breakthrough advanced our understanding of atomic structure and laid the groundwork for further research in nuclear physics and particle physics. The discovery of neutrons, along with protons and electrons, as fundamental constituents of the atomic nucleus remains a cornerstone of modern atomic theory.
Looking for a more dynamic learning experience?
Explore our engaging video lessons and interactive animations that GoPhysics has to offer – your gateway to an immersive physics education!
GCSE Physics Tutorial - The Subdivision of Nucleus Positive Charge: Discovery of Protons
In this tutorial, we will explore how experiments conducted after the Bohr model led to the revolutionary idea that the positive charge of any atomic nucleus could be subdivided into smaller particles. This discovery marked a significant advancement in our understanding of atomic structure and paved the way for further insights into the constituents of the atomic nucleus. Let's delve into the key experiments and ideas that led to the identification of these smaller positively charged particles.
Background: After Niels Bohr proposed his atomic model, which described the arrangement of electrons in discrete energy levels, scientists continued to investigate the structure of the atomic nucleus. They sought to understand the nature of the positively charged nucleus and its role in defining the properties of different elements.
The Discovery of Protons: The key experiments that led to the idea of subdividing the positive charge of the nucleus involved the study of radioactivity and the interaction of particles with matter. In the early 20th century, Ernest Rutherford conducted experiments involving alpha particles, which are positively charged particles emitted during certain types of radioactive decay.
Rutherford's Gold Foil Experiment: In Rutherford's famous gold foil experiment, alpha particles were directed at a thin sheet of gold foil. According to the prevailing Plum Pudding model, the alpha particles were expected to pass through the gold foil with only minor deflections due to the uniform distribution of positive charge in the atom.
Unexpected Results: Contrary to expectations, some of the alpha particles experienced significant deflections, while a few even bounced directly backward. This unexpected outcome indicated that most of the atom's mass and positive charge were concentrated in a tiny, dense region at the center, which Rutherford named the nucleus.
Conclusions: Based on the results of the gold foil experiment and subsequent research, it became clear that the positive charge of the nucleus was not uniformly distributed but was concentrated in individual positively charged particles. These particles were named "protons."
Protons: Elementary Unit of Positive Charge: Protons are elementary particles carrying a positive charge. Each proton has an electric charge of +1 elementary charge, denoted as "e." The charge of one proton is equal in magnitude but opposite in sign to the charge of one electron, which has a charge of -1e.
Atomic Number and Protons: The number of protons present in an atom's nucleus is known as the atomic number (Z). The atomic number defines the identity of the element, as atoms of different elements have different numbers of protons. For example, all carbon atoms have six protons in their nucleus, resulting in a carbon atom having an atomic number of 6.
Electrical Neutrality of Atoms: Atoms are electrically neutral, meaning they have an equal number of protons (positive charge) and electrons (negative charge). The positive charge of the protons is balanced by the negative charge of the electrons, resulting in no net charge for the atom as a whole.
Conclusion: In this tutorial, we have explored how experiments conducted after the Bohr model led to the groundbreaking idea that the positive charge of any atomic nucleus could be subdivided into smaller particles, known as protons. The discovery of protons provided crucial evidence that the nucleus contained individual positively charged entities, each carrying the elementary unit of positive charge. This revelation significantly advanced our understanding of atomic structure and laid the foundation for further research in nuclear physics and quantum mechanics. The identification of protons as one of the fundamental building blocks of matter continues to be a cornerstone in modern atomic theory.
Recalling the Discovery of the Neutron
In the early 20th century, the understanding of atomic structure was evolving rapidly due to groundbreaking experiments and discoveries. One of the significant discoveries was the existence of the neutron, a subatomic particle that plays a crucial role in the composition of atomic nuclei.
The Search for the Neutron: At the time, it was known that atoms were composed of protons, electrons, and a nucleus. However, there were some inconsistencies in the atomic model. For instance, the mass of an atom's nucleus was significantly larger than the combined masses of its protons and electrons. This led scientists to hypothesize the existence of another subatomic particle within the nucleus.
James Chadwick's Experiment: In 1932, British physicist James Chadwick conducted an experiment that provided strong evidence for the existence of the neutron. Chadwick used a technique known as "scattering" to investigate the behaviour of particles when they collided with atoms. He bombarded beryllium atoms with alpha particles, which are positively charged particles commonly emitted during radioactive decay.
Chadwick observed that the scattering of alpha particles by beryllium atoms produced an uncharged particle that had roughly the same mass as a proton. This particle was initially called the "neutral proton" but was later named the "neutron." The discovery of the neutron provided a more complete understanding of atomic nuclei and resolved the inconsistency in the mass of atomic nuclei.
Key Points to Remember:
Neutron's Charge: Unlike protons and electrons, neutrons have no electric charge. They are electrically neutral particles.
Mass of Neutron: The mass of a neutron is slightly larger than that of a proton.
Stability of Nuclei: The presence of neutrons in atomic nuclei helps stabilise them by counteracting the repulsive forces between positively charged protons. Neutrons contribute to the strong nuclear force that holds the nucleus together.
Isotopes: The number of neutrons in an atom's nucleus can vary while keeping the number of protons constant. Atoms of the same element with different numbers of neutrons are called isotopes.
Significance: The discovery of the neutron had a profound impact on the understanding of atomic structure and the behaviour of matter. It paved the way for further research into nuclear physics and led to the development of technologies such as nuclear reactors and nuclear weapons. The neutron's presence and its interactions with other particles play a critical role in nuclear reactions and processes.
In summary, the discovery of the neutron was a milestone in the field of particle physics, contributing to the refined understanding of atomic nuclei and leading to advancements in various scientific and technological applications.
Looking for a more dynamic learning experience?
Explore our engaging video lessons and interactive animations that GoPhysics has to offer – your gateway to an immersive physics education!